BTSMP1 EN12830
EN12830
The control and monitoring of temperatures throughout the cold chain are very important issues with regard to the quality of goods. In order to ensure that food products and products from the pharmaceutical industry are kept at adequate temperatures and that these temperatures are recorded for continuous control purposes, a number of standards must be met.
European regulations require, among other things, the use of recorders that comply with the regulations, which must be regularly checked (Learn more about sensor calibration). These two aspects related to the traceability of the cold chain are the subject of standards called respectively EN12830 for the recorders and EN13486 for their verification.
In this article, we propose a focus on these 2 standards, their scope of application and the legal obligations resulting from them.
Scope of this regulation: where? For whom? Why is it necessary?
In a context of globalization, the storage, transport and distribution of heat-sensitive products are subject to different regulations depending on the country. This article will only deal with the subject of regulations on the European territory. There are many professions that have to comply with the regulations on cold chain traceability and you must make sure of the standards applicable to your branch of activity.
The text of the EN12830 standard gives an example of the fields of activity identified: “Examples of goods that are sensitive to transport, storage and distribution temperatures between -80 °C and +85 °C are refrigerated, frozen and deep-frozen goods, ice cream, fresh and hot food, pharmaceuticals, blood, organs, chemicals, biological materials, electronic and mechanical devices, flowers, plants, bulbs, raw materials and liquids, animals, works of art and furniture. »
The scope of application is very broad, but we focus on 2 major branches of activity in terms of transport and logistics covered by this regulation:
Storage and transport of chilled, frozen and deep-frozen perishable goods. Storage and transport of medical products: pharmaceuticals, blood products, organs and medical samples.
Proprietary solution or open system?
There are many ways to deal with these security and integrity issues, but there are two main approaches:
The first is to ensure the security and integrity of the data through a proprietary system. In short, from the sensor to the data visualization application level. In this case, compliance with the standard is easier to achieve because it is handled at the application level, which has significant computing resources. Nevertheless, this approach requires the exclusive use of the entire manufacturer’s solution: from the temperature sensor to the final application through all the manufacturer’s own communication layers. The second is to ensure data security and integrity through an open system. The temperature sensor itself is designed to meet the security and integrity requirements imposed by the standard. In this case compliance with the standard is more difficult to achieve, as it requires that this be handled at the sensor level, which has limited computing resources. However, this way of doing things allows manufacturers to remain open to any independent system, while ensuring compliance with the EN12830 standard.
How do I know if a recorder complies with the EN12830 standard?
The manufacturer of your recorder must be able to issue a certificate of conformity in its own name. Furthermore, this equipment must have undergone conformity tests carried out by a COFRAC-accredited standardization laboratory. The manufacturer must be able to provide you with the test report from this COFRAC-accredited laboratory. Attention, it is your responsibility to check that the report refers to the standard in force: in this case the 2018 version of EN12830.
Moreover, in France, the Ministry of Agriculture regularly publishes the list of compliant temperature recorders, which can be consulted here. However, this list does not give any information on the version of the standard used for the declaration of conformity: 1999 or 2018. EN13486: verification of the recorder accuracy
Compliance with the EN12830 standard is unfortunately not sufficient. You must also comply with the EN13486 standard! Attention, the current revision of the EN13486 standard dates from 2002 and a CEN technical committee has launched the revision of this standard NF EN 13486.
It is well known that sensors have the unfortunate tendency to drift over time, i.e. the measurement they give can become progressively less accurate after a few years. This is true for all sensors and also for temperature sensors. This is why periodic inspections have been imposed and you must comply with them.
The periodic verification of the recorders is the responsibility of its user, i.e. the carrier and the logistician. Nevertheless, there are some rules to know to organize a certification campaign to EN13486 :
This periodic verification must be done by a COFRAC (Le Comité Français d’Accréditation) accredited body for France. The laboratory you have chosen must be able to give you its “accreditation certificate” issued by COFRAC with these validity dates. You must define the reference temperature(s) for which you want to obtain the periodic verification. Be careful, the cost of certification is often proportional to the number of temperature values to be verified. You need to define the accuracy class for which you want the periodic verification. This class is related to your industry.
What temperature values must you observe?
The definition of the temperature values that you must respect depends on your sector of activity. To help you, if you are in the case of transport of perishable foodstuffs, the Ministry of Agriculture publishes a summary table of storage temperatures to be respected. You can download this documentation here.
Here is an excerpt from the document:
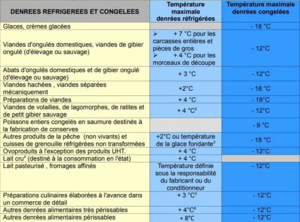
For the transport of pharmaceuticals, blood and samples the 3 most frequently used temperature values are as follows:
- 20°C (range 18 to 24°C)
- 4°C (range 2 to 8°C)
- – 20°C (range-30 to – 18°C).
It is quite frequent in this sector of activity that the recorders are certified for all 3 temperature values simultaneously, which inevitably increases the cost of certification. Attention, this is given for information only and it is important to check the requirements of your sector of activity. What temperature accuracy do you have to respect?
The definition of the temperature accuracy you need to meet depends on your industry and your requirements. For example for the transport of perishable goods your recorder must be accurate to + or – 2°C. It must therefore be class 2 or better (Class 1 for example).
Concerning the transport of pharmaceutical and blood products the recorder temperature class requested and frequently class 1 (+ or – 1°C accuracy) in the negative temperature ranges and class 0.5 (+ or – 0.5°C accuracy) in the positive ranges.
Be careful, it is up to you to define the class you wish to verify and the cost of the certification is all the higher the more precise the requested class is. How often should you have your recorder checked?
The text of the standard states that “the frequency of testing depends on the requirements of the user, taking into account the manufacturer’s specifications”. Nevertheless, it gives some recommendations: “It is recommended, however, that the manufacturer or workshops authorized by the manufacturer or the approved verification services carry out an inspection each year, when the temperature recorders and thermometers have been used during this period. » How do you know if your recorder complies with the EN13486 standard?
The laboratory that performed the periodic verification shall be able to provide a verification report or calibration report for each recorder or sensor with the date of the report. In addition, the recorder itself shall be visibly marked with the fact that it has been verified and the date of verification.
Attachments
 |
You can the find PDF version of the EN12830 Certification here.
